A collaboration that originated as a CTP-network gave us this nice paper. Congratullations Jonathan!
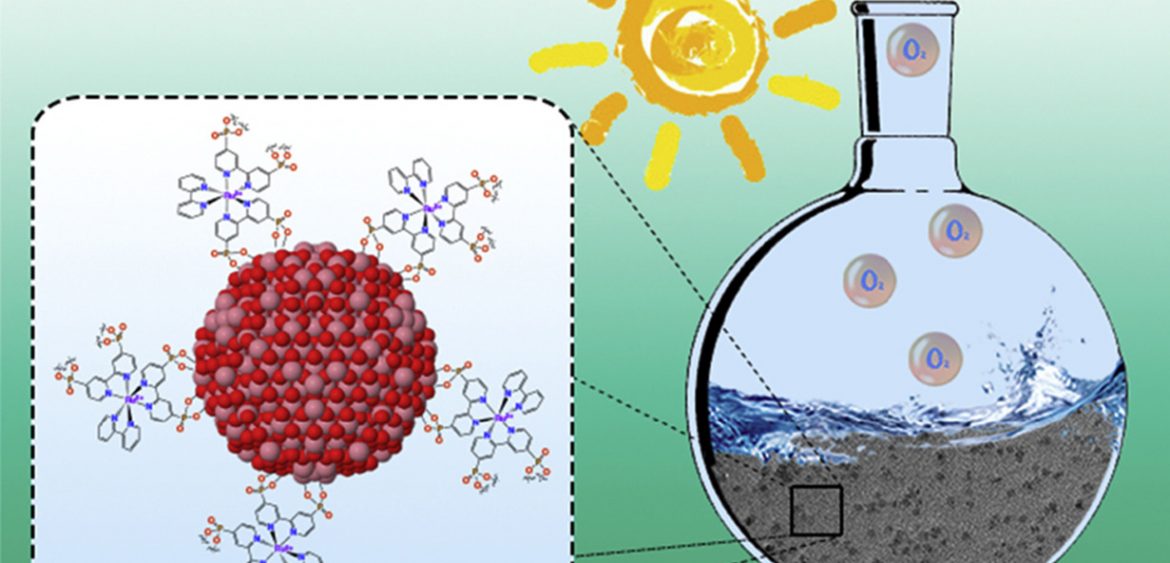
Cobalt nanoparticles (NPs) have been prepared by hydrogenation of the organometallic complex [Co(eta(3)-C8H13)(eta(4)-C8H12)] in 1-heptanol in the absence of any other stabilizer and then transformed into Co3O4 NPs using mild oxidative reaction conditions. After deposition onto glassy carbon rotating disk electrodes, the electrocatalytic performance of the Co3O4 NPs in water oxidation has been tested in 1M NaOH. The activity has been benchmarked with that of state-of-the-art Co3O4 NPs through electrochemically-active surface area (ECSA) and specific current density measurements. Furthermore, the covalent grafting of photosensitive polypyridyl-based RuII complexes onto the surface of Co3O4 NPs afforded hybrid nanostructured materials able to photo-oxidize water into O-2, while steady-state and time-resolved spectroscopic measurements gave some further insight into kinetics and pertinent reaction steps following excitation. These first-row transition metal oxide hybrid nanocatalysts display better catalytic performance than simp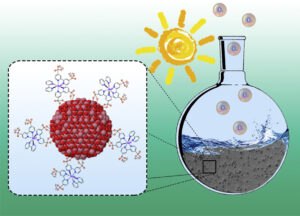 le mixtures of non-grafted photosensitizers and Co3O4 NPs, thus evidencing the advantage of the direct coupling between the two entities for the photo-induced water oxidation reaction. (c) 2018 Elsevier Ltd. All rights reserved.
le mixtures of non-grafted photosensitizers and Co3O4 NPs, thus evidencing the advantage of the direct coupling between the two entities for the photo-induced water oxidation reaction. (c) 2018 Elsevier Ltd. All rights reserved.
Access full text here.






Sorry, the comment form is closed at this time.