 Surprised to see a giant KIE in an organometallic catalytic reaction? Check our explanation now published in ACIE (2022).
Surprised to see a giant KIE in an organometallic catalytic reaction? Check our explanation now published in ACIE (2022).
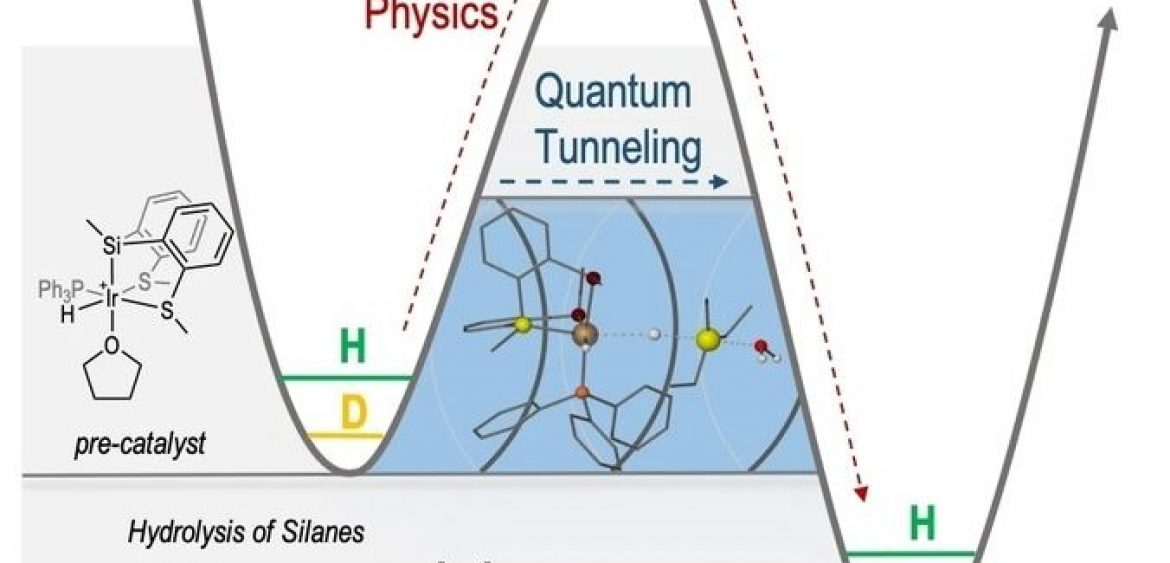
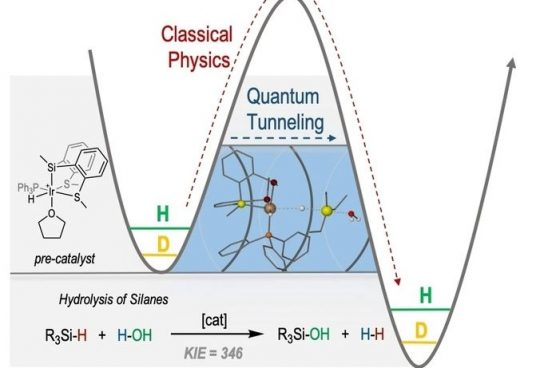
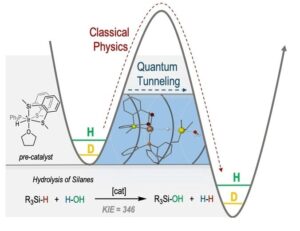
An unprecedented quantum tunneling effect has been observed in catalytic Si−H bond activations at room temperature. The cationic hydrido-silyl-iridium(III) complex, {Ir[SiMe(o-C6H4SMe)2](H)(PPh3)(THF)}[BArF4], has proven to be a highly efficient catalyst for the hydrolysis and the alcoholysis of organosilanes. When triethylsilane was used as a substrate, the system revealed the largest kinetic isotopic effect (KIESi−H/Si−D=346±4) ever reported for this type of reaction. This unexpectedly high KIE, measured at room temperature, together with the calculated Arrhenius preexponential factor ratio (AH/AD=0.0004) and difference in the observed activation energy [(E −E
−E )=34.07 kJ mol−1] are consistent with the participation of quantum tunneling in the catalytic process. DFT calculations have been used to unravel the reaction pathway and identify the rate-determining step. Aditionally, isotopic effects were considered by different methods, and tunneling effects have been calculated to be crucial in the process.
)=34.07 kJ mol−1] are consistent with the participation of quantum tunneling in the catalytic process. DFT calculations have been used to unravel the reaction pathway and identify the rate-determining step. Aditionally, isotopic effects were considered by different methods, and tunneling effects have been calculated to be crucial in the process.